Introduction Heat Transfer
Heat transfer is that science which seeks to predict the energy transfer which may take place between material bodies as a result of a temperature difference.
Thermodynamics teaches that this energy transfer is defined as heat. The science of heat transfer seeks not merely to explain how heat energy may be transferred, but also to predict the rate at which the exchange will take place under certain specified conditions. The fact that a heat transfer rate is the desired objective of an analysis points out the difference between heat transfer and thermodynamics.
Thermodynamics deals with system in equilibrium; it may be used to predict the amount of energy required to change a system from one equilibrium state to another; it may not be used to predict how fast a change will take place since the system is not in equilibrium during the process. Heat transfer supplements the first and second principles of thermodynamics by providing additional experimental rules which may be used to establish energy transfer rates. As in the science of thermodynamics, the experimental rules used as a basis of the subject of heat transfer are rather simple and easily expanded to encompass a variety of practical situations.
As an example of the different kinds of problems which are treated by thermodynamics and heat transfer, consider the cooling of a hot steel bar which is placed in a pail of water. Thermodynamics may be used to predict the final equilibrium temperature of the steel bar-water combination. Thermodynamics will not tell us how long it takes to reach this equilibrium condition or what the temperature of the bar will be after a certain length of time before the equilibrium condition is attained. Heat transfer may be used to predict the temperature of both the bar and the water as a function of time.
Most readers will be familiar with the terms used to denote the three modes of heat transfer: conduction, convection and radiation. In this chapter we seek to explain the mechanism of these modes qualitatively so that each may be considered in its proper perspective. Subsequent chapters treat the three types of heat transfer in detail.
1. Conduction Heat Transfer
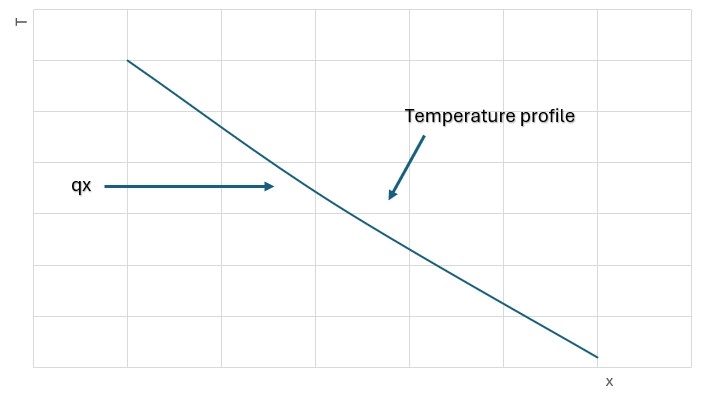
When a temperature gradient exists in a body, experience has shown that there is an energy transfer from the high temperature region to the low temperature region. We say that the energy is transferred by conduction and that the heat transfer rate per unit area is proportional to the normal temperature gradient:
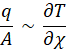
When the proportionality constant is inserted,
q =
where q is the heat transfer rate and ǝT/ǝx is the temperature gradient in the direction of the heat flow. The positive constant k is called the thermal conductivity of the material, and the minus sign is inserted so that the second principle of thermodynamics will be satisfied; i.e., heat must flow downhill on the temperature scale, as indicated in the coordinate system of Fig. 1-1, equation (1-!1) is called Fourier’s law of heat conduction after the French mathematical physicist Joseph Fourier, who made very significant contribution to the analytical treatment of conduction heat transfer. It is important to note that eq. 1-1 is the defining equation for the thermal conductivity and that k has the units of watts per meter per Celsius degree in a typical system of units in which the heat flow is expressed in watts. We now set ourselves the problem of determining the basic equation which governs the transfer of heat in a solid, using eq 1-1 as a starting point.